Cf4 at room temperature. Both CCl4 and CF4 are non-polar molecules.

Cf4 Capture And Separation Of Cf4 Sf6 And Cf4 N2 Fluid Mixtures Using Selected Carbon Nanoporous Materials And Metal Organic Frameworks A Computational Study Acs Omega
In our model of an inductively coupled CF4 plasma in the GEC Cell at 1 Wcm 3 neutral.

. Give the molecular shape around the boron atom in BCl 3 and the nitrogen atom in NCl 3 and explain why they. CF4 SiH4 PH3 NH2OH. OK electro-negativity difference between Carbon and Fluoride 15 is FAR greater than Carbon and Brom 03.
Bonding of glass nanofluidic chips at room temperature by a one-step surface activation using an O2CF4 plasma treatment Lab Chip. A fluorocarbon CF4 has a critical temperature of 457 C and a critical pressure of 37 atm. The pressure dependences of the intramolecular vibrational modes suggest phase transitions from phase III to IV at 89 GPa and from phase IV to V at 136 GPa.
A is highly flammable. CF4 g CCl4 l CBr4 s. Be notified when an answer is posted.
The space group of phase III was determined as P 21 c. CF4 CCl4and CBr4 is liquid gas and solid. Now here is methane CH₄.
It is believed that the data are accurate to 01 at 25C and to 03 at 600C. 2013 Mar 21. Answer to A fluorocarbon CF4 has a critical temperature of 457.
Explain why the bond angles in BCl3 and NCl3 are different. Are there any conditions under which this compound can be a liquid at. Nanostructure-friendly and high pressure-resistant bonding method performed at room temperature RT 25 C for glass nanofluidic chips using a one-step surface activation process with an O2.
The pressure-induced phase transformation of solid CF4 at room temperature was studied with x-ray and Raman scattering experiments. Here is assignment. Here is carbon tetrachloride CCl₄.
Interactions between such molecules are usually governed by van der Waals forces which generally increase with increasing molecular size and mass. The space group of phase III was determined as P 21 c. What is the room temperature of CF4.
Is CF4 a gas at room temperature. Thus CCl4 would be expected to have a somewhat higher bp than CF4 has. The highest critical temperature.
Further more CF4 is a gas at room temperature while H20 is a liquid at room temperature. Which of the following substances is most likely to be a liquid at room temperature. One of these substances is a liquid at room temperature.
B covalent compounds are all gases but ionic compounds may be solids liquids or gases. Chloroform which is indeed a liquid at room temperature ϑ b 612 C is C H C l X 3 or trichloromethane. The smallest units both compounds are made of CCl₄ and CH₄ molecules that is have the same molecular geometry - they are both tetrahedra.
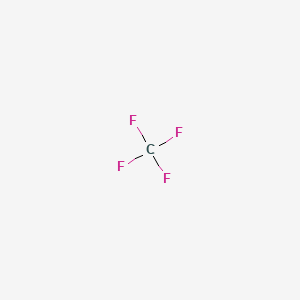
CF4 comprises a Carbon atom surrounded by four Fluorine atoms. At room temperature it is a colorless odorless nonflammable and compressible gas with high volatility. The paper contains new measurements of the low-density vicosity of CH 4 25200C CF 4 25600C and SF 6 25300C.
One of these substances is a liquid at room temperature. Obtained at room temperature showed that extraction efficiency in NeCF4 9010 is very close to the one measured in pure CF4 22. A A volatile liquid is one that _____.
CF4 SiH4 PH3 NH2OH. Answer 1 of 7. Custom TAMU CHEM 102 Chemistry for Engineering Students Volume I 8th Edition Edit edition Solutions for Chapter 12 Problem 53SCQ.
Answer 1 of 2. AFormaldehyde H2CO bfluoromethane CH3F chydrogen cyanide HCN dhydrogen peroxide H2O2 ehydrogen sulfide H2S. This is actually a very good question.
Both are polar substances but CF4 is far more polar than CBr4 due to the. You need to substitute three hydrogens with chlorine atoms to create chloroform. One of these substances is a liquid at room temperature.
Use what you have learned about molecular geometry polarity and intermolecular forces to explain why CO2 is a gas at room temperature but H2O is a liquid at room temperature. In general at room temperature a ionic compounds are all solids but covalent compounds may be solids liquids or gases. A CBr4 B CCl4 C CF4 D CH4 E H2.
It is a gas at room temperature with a boiling point ϑ b 238 C. Therefore these are very different structures. C H X 3 C l is not chloroform but methyl chloride or chloromethane.
When it comes to PCB laminates there is FR-4 and there is FR-4 and other types. At room temperature which substance of these. A CH3OH b CF4 c SiH4 d CO2.
The data can be interpolated and probably also extrapolated with the aid of our extensive law of corresponding states.

Cf4 Capture And Separation Of Cf4 Sf6 And Cf4 N2 Fluid Mixtures Using Selected Carbon Nanoporous Materials And Metal Organic Frameworks A Computational Study Acs Omega

A A Schematic Of Multilayer Graphene During Cf4 Plasma Treatment B Download Scientific Diagram
Is Cf4 Polar Or Nonpolar Quora

Lower Curve Shows Cf4 Partial Pressure The Upper Curve Indicates The Download Scientific Diagram

Cf4 Molecular Geometry Bond Angles Electron Geometry Carbon Tetrafluoride

Thermodynamic Equilibrium Composition Of A Nf3 B Sf6 C Cf4 D Download Scientific Diagram

Important Reasons To Ditch The Teflon Wellness Mama Safest Cookware Greenpan
0 comments
Post a Comment